Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Potential of Liquid Biopsy in Cancer Diagnosis and Monitoring of Treatment Response
*Corresponding author:Lucy Mohapatra, Amity Institute of Pharmacy, Amity University Uttar Pradesh, India.
Received:May 05, 2023; Published:May 11, 2023
DOI: 10.34297/AJBSR.2023.18.002521
Abstract
Liquid biopsy, a non-invasive method of analyzing cancer biomarkers in blood and other bodily fluids, has emerged as a promising approach for cancer diagnosis and monitoring of treatment response. The three main types of liquid biopsy include Circulating Tumor Cells (CTCs), Cell-Free DNA (cfDNA), and Extracellular Vesicles (EVs). Liquid biopsy offers several advantages over traditional tissue biopsy, including minimal invasiveness, ability to monitor disease progression in real-time, and potential for early cancer detection. However, challenges and limitations associated with liquid biopsy remain, such as the need for standardized protocols and assays, and the potential for false positives and negatives. Despite these challenges, liquid biopsy has shown great promise in clinical applications, such as monitoring of treatment response and resistance, and the identification of actionable mutations for targeted therapy. In conclusion, liquid biopsy holds great potential for improving cancer diagnosis and management, and ongoing research efforts will continue to refine and expand its clinical applications.
Keywords: Liquid biopsy, Cancer diagnosis, Treatment monitoring, Biomarkers, Personalized medicine
Introduction to Liquid Biopsy in Cancer Diagnosis and Monitoring
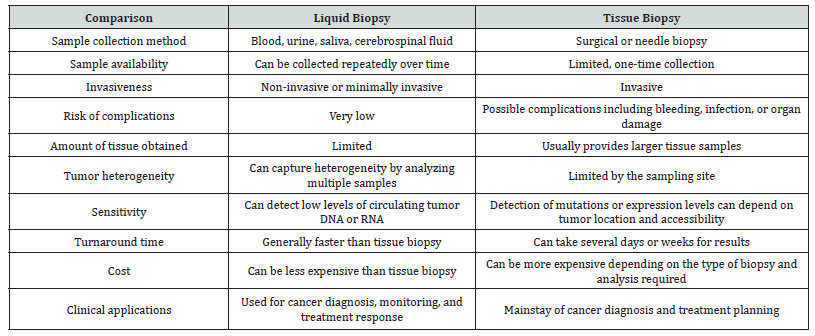
Cancer is one of the leading causes of death worldwide, and early diagnosis is critical for successful treatment and improved patient outcomes. Traditional diagnostic methods for cancer involve invasive tissue biopsies, which can be painful, risky, and expensive. In recent years, liquid biopsy has emerged as a promising alternative to tissue biopsy, offering a non-invasive and minimally invasive method for cancer diagnosis and monitoring of treatment response [1].
Liquid biopsy involves the analysis of various biomolecules, including Circulating Tumor Cells (CTCs), Circulating Tumor DNA (ctDNA), Extracellular Vesicles (EVs), and Circulating MicroRNAs (miRNAs) in blood or other body fluids [2]. CTCs are tumor cells that have detached from the primary tumor and are circulating in the bloodstream. ctDNA is released into the bloodstream by dying cancer cells and can be used to detect tumor-specific mutations. EVs are small lipid-bilayer vesicles secreted by cells, including cancer cells, and contain various biomolecules, including proteins, lipids, and nucleic acids [3]. miRNAs are small non-coding RNAs that regulate gene expression and have been shown to be dysregulated in cancer. One of the main advantages of liquid biopsy is its noninvasive nature, which eliminates the need for invasive tissue biopsies. This makes it a safer and less painful method for cancer diagnosis and monitoring of treatment response. In addition, liquid biopsy is a minimally invasive method that can be performed repeatedly, allowing for the monitoring of disease progression and treatment response over time.
Liquid biopsy has shown great potential in cancer diagnosis and monitoring of treatment response. Several studies have demonstrated the utility of liquid biopsy in the early detection of cancer, particularly in the detection of early-stage lung cancer. For example, a study published in the New England Journal of Medicine found that liquid biopsy was able to detect lung cancer with a sensitivity of 72% and a specificity of 90% in patients with stage I to III lung cancer. In addition, liquid biopsy has shown promise in monitoring treatment response in cancer patients. For example, a study published in Cancer Research found that changes in ctDNA levels could be used to monitor treatment response in patients with metastatic breast cancer. Another study published in JAMA Oncology found that changes in CTC levels could be used to monitor treatment response in patients with metastatic prostate cancer.
Liquid biopsy also has the potential to overcome the limitations of tissue biopsy in detecting tumor heterogeneity. Tumor heterogeneity refers to the presence of different subpopulations of cancer cells within a single tumor. Tissue biopsy samples only a small portion of the tumor, which may not be representative of the entire tumor. Liquid biopsy, on the other hand, allows for the analysis of multiple biomarkers from different regions of the tumor, providing a more comprehensive view of tumor heterogeneity. Despite its potential, liquid biopsy still faces several challenges that must be addressed. One of the main challenges is the detection of low levels of circulating biomarkers, which requires highly sensitive and specific detection methods. In addition, the lack of standardization in sample collection, processing, and analysis can lead to variability in results and limit the clinical utility of liquid biopsy. Hence liquid biopsy has emerged as a promising alternative to tissue biopsy in cancer diagnosis and monitoring of treatment response. Its non-invasive nature, ability to monitor disease progression and treatment response over time, and potential to overcome the limitations of tissue biopsy make it an attractive option for cancer patients. However, further research is needed to address the challenges associated with liquid biopsy and to establish standardized protocols for sample collection, processing, and analysis to ensure its clinical utility.
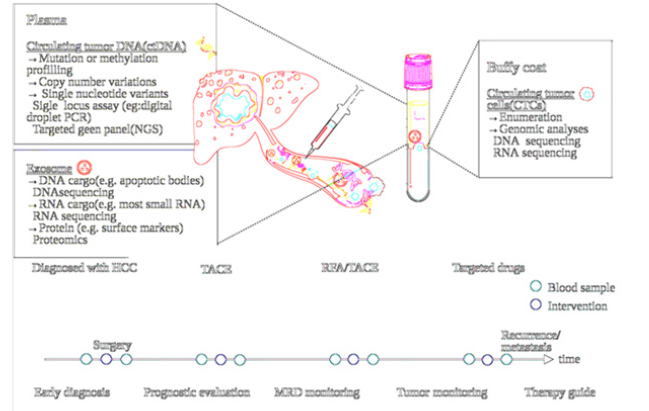
Liquid biopsy is a minimally invasive approach that allows for the detection and monitoring of cancer using a variety of biofluids, including blood, urine, and cerebrospinal fluid. This technique provides a means of accessing and analyzing cancerderived material, such as Circulating Tumor Cells (CTCs), Cell-Free DNA (cfDNA), and Extracellular Vesicles (EVs), that are shed by tumors and released into the bloodstream or other bodily fluids. Liquid biopsy has several advantages over traditional tissue biopsy, including its ability to provide real-time information on cancer progression and treatment response, its potential for early detection of cancer, and its ability to identify the emergence of drug resistance. Furthermore, liquid biopsy is a less invasive and more convenient method for patients, as it eliminates the need for repeat tissue biopsies. Despite these advantages, liquid biopsy also faces several challenges and limitations, such as the need for standardized assays, sample heterogeneity, and the potential for false positives or negatives. We will provide an overview of liquid biopsy in cancer diagnosis and monitoring, highlighting its potential as a tool for personalized cancer management (Table 1).
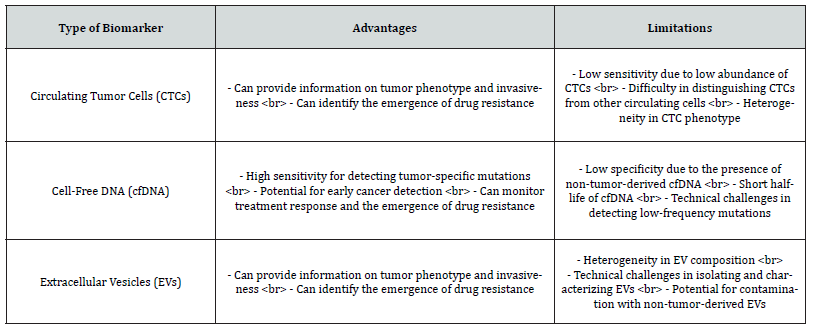
Table 1:It compares the advantages and limitations of the three main types of biomarkers that can be detected using liquid biopsy in cancer diagnosis and monitoring: circulating tumor cells (CTCs), cell-free DNA (cfDNA), and extracellular vesicles (EVs). It provides a concise and organized way to present this information, making it easy for readers to compare the different types of biomarkers.
Types of Liquid Biopsy
Liquid biopsy refers to the non-invasive approach of detecting cancer through the analysis of tumor-derived material present in various biofluids. The three main types of biomarkers that can be detected using liquid biopsy are Circulating Tumor Cells (CTCs), Cell-Free DNA (cfDNA), and Extracellular Vesicles (EVs).
CTCs are tumor cells that have detached from the primary tumor and entered the bloodstream. They can provide valuable information on tumor phenotype and invasiveness, as well as identify the emergence of drug resistance. However, CTCs are present at low frequencies in the blood and can be difficult to distinguish from other circulating cells. cfDNA refers to the fragments of DNA that are released by apoptotic or necrotic cells, including tumor cells, into the bloodstream. cfDNA can be used to detect tumor-specific mutations, monitor treatment response, and identify the emergence of drug resistance. However, cfDNA can also be derived from non-tumor cells and may not be specific to cancer
EVs are small vesicles that are released by cells, including tumor cells, into the bloodstream. They can provide information on tumor phenotype and invasiveness, as well as identify the emergence of drug resistance. However, EVs are heterogeneous in composition and can be difficult to isolate and characterize.
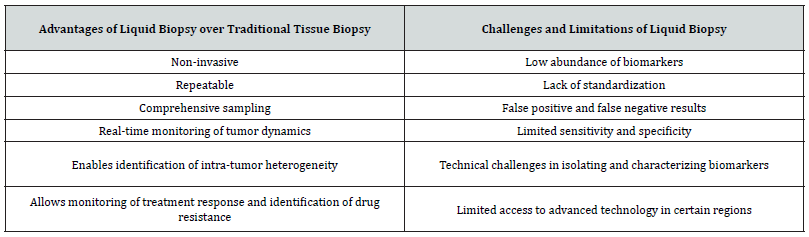
Each type of liquid biopsy has its own advantages and limitations. By combining the analysis of different types of biomarkers, liquid biopsy has the potential to provide a comprehensive picture of the tumor and its response to therapy, enabling personalized cancer management. Liquid biopsy offers several advantages over traditional tissue biopsy, including non-invasiveness, repeatability, comprehensive sampling, real-time monitoring of tumor dynamics, and the ability to identify intra-tumor heterogeneity [4]. Additionally, liquid biopsy enables monitoring of treatment response and identification of drug resistance, which can inform clinical decision-making. However, liquid biopsy also faces several challenges and limitations, including the low abundance of biomarkers, lack of standardization, potential for false positive and false negative results, limited sensitivity and specificity, technical challenges in isolating and characterizing biomarkers, and limited access to advanced technology in certain regions. Despite these challenges, liquid biopsy has the potential to revolutionize cancer diagnosis and monitoring by enabling personalized cancer management (Table 2).
Liquid Biopsy for Early Cancer Detection and Diagnosis
Liquid biopsy has emerged as a promising approach for early cancer detection and diagnosis, as it enables the non-invasive analysis of tumor-derived material in various biofluids. The detection of cancer at an early stage can significantly improve patient outcomes, as it allows for earlier intervention and potentially curative treatment options. Several studies have demonstrated the potential of liquid biopsy for early cancer detection and diagnosis. For example, the detection of mutations in cfDNA has been shown to be effective for the early detection of lung cancer, colorectal cancer, and pancreatic cancer. Similarly, the analysis of CTCs has been shown to be effective for the early detection of breast cancer and prostate cancer.
However, the clinical utility of liquid biopsy for early cancer detection and diagnosis is still under investigation, and further research is needed to validate the sensitivity and specificity of liquid biopsy-based tests. Additionally, the development of standardized protocols and quality control measures is necessary to ensure the accuracy and reproducibility of liquid biopsy-based tests. Despite these challenges, the potential of liquid biopsy for early cancer detection and diagnosis is significant, and it has the potential to revolutionize cancer screening and detection by enabling noninvasive, convenient, and cost-effective testing.
Liquid Biopsy based bio markers for cancer
Liquid biopsy has shown great potential for the detection and monitoring of cancer based on various biomarkers in blood or other body fluids. These biomarkers include Circulating Tumor Cells (CTCs), Circulating Tumor DNA (ctDNA), Extracellular Vesicles (EVs), and Circulating MicroRNAs (miRNAs), among others [5-8]. Here are some of the potential applications of liquid biopsy-based biomarkers for cancer:
Early Detection of Cancer
Liquid biopsy-based biomarkers have shown promise in the early detection of cancer, particularly in the detection of early-stage cancer. For example, ctDNA can be used to detect tumor-specific mutations in early-stage lung cancer, while CTCs can be used to detect early-stage breast cancer [9].
Monitoring of Treatment Response
Liquid biopsy-based biomarkers can be used to monitor treatment response in cancer patients. For example, changes in ctDNA levels can be used to monitor treatment response in patients with metastatic breast cancer, while changes in CTC levels can be used to monitor treatment response in patients with metastatic prostate cancer [10].
Prognostic Value
Liquid biopsy-based biomarkers can also provide prognostic information in cancer patients. For example, high levels of CTCs and ctDNA have been associated with poor prognosis in various types of cancer, including breast, lung, and colorectal cancer [11].
Tumor Heterogeneity
Liquid biopsy-based biomarkers can help overcome the limitations of tissue biopsy in detecting tumor heterogeneity. For example, by analyzing multiple biomarkers from different regions of the tumor, liquid biopsy can provide a more comprehensive view of tumor heterogeneity [12].
Minimal Residual Disease Detection
Liquid biopsy-based biomarkers can also be used to detect Minimal Residual Disease (MRD) in cancer patients. MRD refers to the presence of small amounts of cancer cells that remain after treatment, which can lead to disease recurrence. Liquid biopsybased biomarkers can detect MRD earlier than traditional imaging methods, allowing for earlier intervention and improved patient outcomes [13].
Personalized Medicine
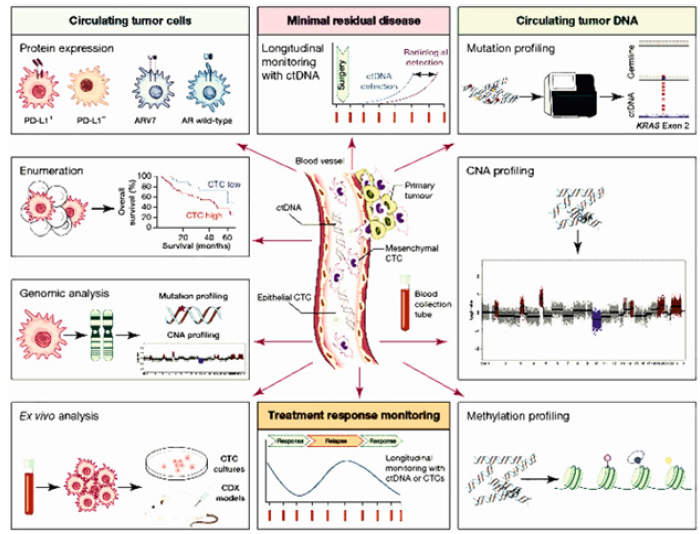
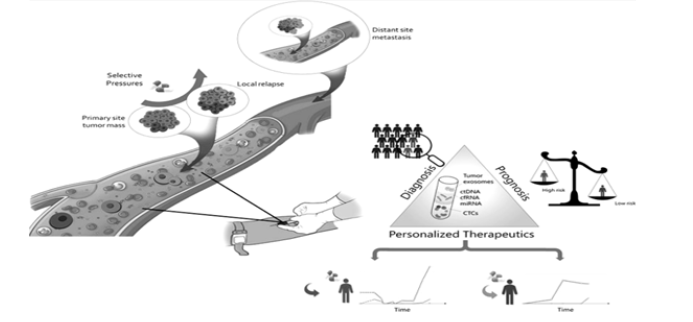
Liquid biopsy-based biomarkers can also be used to guide personalized cancer treatment. For example, by analyzing ctDNA, oncologists can identify specific mutations that are driving tumor growth and select targeted therapies that are most likely to be effective (Figure 1).
Liquid Biopsy for Monitoring Treatment Response and Resistance
Liquid biopsy has shown immense potential for monitoring treatment response and resistance in cancer patients. Traditional methods for monitoring treatment response, such as imaging scans and tissue biopsies, are often invasive, expensive, and time-consuming. In contrast, liquid biopsy is a non-invasive and convenient approach for monitoring treatment response, as it enables the analysis of tumor-derived material in various biofluids. One of the main biomarkers used for monitoring treatment response and resistance in liquid biopsy is ctDNA. The analysis of ctDNA can provide real-time information on tumor burden and the emergence of resistance mutations. Additionally, the analysis of CTCs and EVs can provide information on tumor phenotype and invasiveness, as well as the emergence of drug resistance (Figure 2).
Several studies have demonstrated the potential of liquid biopsy for monitoring treatment response and resistance in cancer patients. For example, the analysis of ctDNA has been shown to be effective for monitoring treatment response in patients with lung cancer, breast cancer, and colorectal cancer. Similarly, the analysis of CTCs has been shown to be effective for monitoring treatment response in patients with prostate cancer and breast cancer. However, liquid biopsy also faces several challenges for monitoring treatment response and resistance. For example, the low abundance of biomarkers in biofluids and the lack of standardization in liquid biopsy protocols can impact the accuracy and reproducibility of liquid biopsy-based tests [14-16].
Despite these challenges, liquid biopsy has the potential to revolutionize cancer management by enabling personalized treatment strategies based on the real-time monitoring of treatment response and resistance. Ongoing research and development in liquid biopsy-based tests are expected to further improve the sensitivity and specificity of these tests for monitoring treatment response and resistance..
Clinical Applications of Liquid Biopsy in tumor management
Liquid biopsy has the potential to play a significant role in tumor management, including diagnosis, treatment, and monitoring of cancer. Here are some ways in which liquid biopsy can be used in tumor management.
Diagnosis
Liquid biopsy-based biomarkers can help diagnose cancer by detecting specific biomolecules that are released by cancer cells into the bloodstream or other bodily fluids. For example, ctDNA and CTCs can be used to identify specific mutations that are indicative of cancer [18].
Treatment Selection
Liquid biopsy can help select the most appropriate treatment for cancer patients by identifying specific mutations that are driving tumor growth. For example, by analyzing ctDNA, oncologists can identify mutations that are responsive to targeted therapies and select treatments that are most likely to be effective [19].
Monitoring of Treatment Response
Liquid biopsy-based biomarkers can be used to monitor treatment response in cancer patients. For example, changes in ctDNA levels can be used to monitor treatment response in patients with metastatic breast cancer, while changes in CTC levels can be used to monitor treatment response in patients with metastatic prostate cancer [20].
Detection of Resistance
Liquid biopsy-based biomarkers can be used to detect resistance to cancer treatments. For example, by analyzing ctDNA, oncologists can detect mutations that confer resistance to targeted therapies, allowing for early intervention and a switch to alternative treatments [21].
Minimal Residual Disease Detection
Liquid biopsy-based biomarkers can be used to detect Minimal Residual Disease (MRD) in cancer patients. MRD refers to the presence of small amounts of cancer cells that remain after treatment, which can lead to disease recurrence. Liquid biopsybased biomarkers can detect MRD earlier than traditional imaging methods, allowing for earlier intervention and improved patient outcomes [22].
Prognostic Value
Liquid biopsy-based biomarkers can provide prognostic information in cancer patients. For example, high levels of CTCs and ctDNA have been associated with poor prognosis in various types of cancer, including breast, lung, and colorectal cancer [23].
Tumor Heterogeneity
Liquid biopsy-based biomarkers can help overcome the limitations of tissue biopsy in detecting tumor heterogeneity. By analyzing multiple biomarkers from different regions of the tumor, liquid biopsy can provide a more comprehensive view of tumor heterogeneity [24].
Clinical Applications of Liquid Biopsy in Cancer Management
Liquid biopsy has several clinical applications in cancer management, including:
a) Early Detection and Diagnosis
Liquid biopsy can be used for the early detection and diagnosis of cancer, enabling earlier intervention and potentially curative treatment options [25].
b) Treatment Selection and Monitoring
Liquid biopsy can be used to guide treatment selection by identifying actionable mutations and monitoring treatment response and resistance [26].
c) Prognosis and Monitoring of Disease Progression
Liquid biopsy can provide information on tumor burden, invasiveness, and the emergence of resistance mutations, enabling clinicians to make informed decisions about disease management [27].
d) Minimal Residual Disease Detection
Liquid biopsy can be used to detect minimal residual disease, which is a small number of cancer cells that remain after treatment and can cause relapse [28].
e) Monitoring For Cancer Recurrence
Liquid biopsy can be used to monitor cancer recurrence and guide surveillance strategies for patients who have completed treatment [29].
f) Research and Drug Development
Liquid biopsy can be used in research and drug development to identify new biomarkers and therapeutic targets, as well as to test the efficacy of new drugs in clinical trials (Figure 3).
Liquid biopsy has the potential to revolutionize cancer management by enabling non-invasive, convenient, and costeffective testing for early detection, treatment selection and monitoring, prognosis, and disease surveillance.
Recent Drugs in Pipeline
Some examples of promising drugs that were in the pipeline are:
a) Sacituzumab govitecan
A novel antibody-drug conjugate that targets Trop-2, a protein that is overexpressed in multiple types of cancer, including breast, lung, and bladder cancer. In April 2021, the US Food and Drug Administration (FDA) approved Sacituzumab govitecan for the treatment of metastatic triple-negative breast cancer [30].
b) Sotorasib
A targeted therapy that inhibits KRAS G12C, a driver mutation found in approximately 13% of Non-Small Cell Lung Cancer (NSCLC) cases. In May 2021, the FDA granted accelerated approval to Sotorasib for the treatment of locally advanced or metastatic NSCLC with KRAS G12C mutation [31].
c) Teclistamab
A bispecific antibody that targets both BCMA and CD3, two proteins found on the surface of multiple myeloma cells and T cells, respectively. In August 2021, the FDA granted breakthrough therapy designation to Teclistamab for the treatment of relapsed or refractory multiple myeloma.
d) Amivantamab
A bispecific antibody that targets both EGFR and MET, two proteins that are frequently overexpressed or mutated in non-small cell lung cancer. In May 2021, the FDA granted accelerated approval to Amivantamab for the treatment of patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations.
e) Zanidatamab
A bispecific antibody that targets both HER2 and HER3, two proteins that are overexpressed in multiple types of cancer, including breast, gastric, and ovarian cancer. In August 2021, the FDA granted breakthrough therapy designation to Zanidatamab for the treatment of patients with HER2-positive metastatic breast cancer who have received prior HER2-targeted therapies [32].
Future Directions and Opportunities in Liquid Biopsy Research
The field of liquid biopsy is rapidly evolving, and there are several future directions and opportunities for research that could further advance the clinical utility of liquid biopsy in cancer management:
f) Multi-omics analysis
Integrating the analysis of multiple types of biomarkers, such as ctDNA, CTCs, and EVs, could provide a more comprehensive understanding of tumor biology and treatment response.
g) Development of novel biomarkers
The discovery and validation of new biomarkers in liquid biopsy, such as RNA and proteins, could enhance the sensitivity and specificity of liquid biopsy-based tests [33].
h) Standardization and quality control
The establishment of standardized protocols and quality control measures is essential for ensuring the accuracy and reproducibility of liquid biopsy-based tests [34].
i) Large-scale clinical validation studies
Large-scale clinical validation studies are needed to demonstrate the clinical utility of liquid biopsy-based tests and establish their role in cancer management [35].
j) Integration with other technologies
Combining liquid biopsy with other technologies, such as imaging and artificial intelligence, could enhance the accuracy and clinical utility of liquid biopsy-based tests.
k) Patient stratification and personalized medicine
Liquid biopsy could be used to stratify patients based on their tumor biology and treatment response, enabling personalized treatment strategies [36].
l) Development of non-cancer applications
Liquid biopsy could have potential applications beyond cancer, such as in infectious diseases and autoimmune disorders [37].
Liquid biopsy is a rapidly evolving field with great potential for improving cancer management. Future research and development in liquid biopsy could further enhance its clinical utility and revolutionize cancer care.
Conclusion and Implications for Clinical Practice
In conclusion, liquid biopsy is a promising and rapidly evolving approach for cancer diagnosis, monitoring, and management. Its non-invasive and convenient nature, coupled with its potential for early detection, treatment selection and monitoring, and disease surveillance, make it an attractive option for clinical practice. However, there are still challenges and limitations that need to be addressed, such as the need for standardized protocols and quality control measures, the development of novel biomarkers, and largescale clinical validation studies. Despite these challenges, liquid biopsy holds great potential for transforming cancer care and improving patient outcomes.
The implications for clinical practice are significant. Liquid biopsy can provide clinicians with real-time information on tumor biology and treatment response, enabling more informed decisionmaking and personalized treatment strategies. It can also reduce the need for invasive tissue biopsies, which can be costly, timeconsuming, and associated with potential complications. However, it is important to note that liquid biopsy-based tests should not replace traditional tissue biopsy in all cases, as tissue biopsy remains the gold standard for definitive diagnosis and characterization of tumors. So, the development and integration of liquid biopsy-based tests into clinical practice could have a significant impact on cancer management, leading to earlier detection, more accurate diagnosis, improved treatment selection and monitoring, and better patient outcomes. The continued research and development in this field are essential for realizing the full potential of liquid biopsy in cancer care.
Acknowledgements
None.
Conflict of Interest
None.
References
- O Leary B, Finn RS, Turner NC (2016) Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 13(7): 417-430.
- Pantel K, Alix Panabières C (2019) Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol 16(7): 409-424.
- Cabel L, Riva F, Servois V, Livartowski A, Daniel C, et al. (2017) Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol 28(8): 1996-2001
- Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, et al. (2008) Circulating mutant DNA to assess tumor dynamics. Nat Med 14(9): 985-990.
- Abbosh C, Birkbak NJ, Wilson GA, Jamal Hanjani M, Constantin T, et al. (2017) Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 545(7655): 446-451.
- Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J (2016) Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell 164(1-2): 57-68.
- Cheng F, Su L, Qian C (2016) Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget 7(30): 48832-48841.
- Thierry AR, Mouliere F, El Messaoudi S, Mollevi C, Lopez Crapez E, et al. (2014) Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med 20(4): 430-435.
- Best MG, Sol N, Kooi I, Tannous J, Westerman BA, et al. (2015) RNA-seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell 28(5): 666-676.
- Park JY, Lee HJ, Kang SY, Park IA, Lee KS (2018) Cell-free DNA derived from different tumor types exhibits a different fragment size pattern. Cancer Res Treat 50(2): 492-500.
- Chae YK, Davis AA, Carneiro BA, Chandra S, Mohindra N, et al. (2016) Concordance and discordance of genomic alterations in tissue and circulating tumor DNA in metastatic melanoma. Clin Cancer Res 22(17): 4570-4578.
- Huang X, Gao P, Sun J, Chen X, Song Y, et al. (2020) Highly sensitive and specific analysis of cancer mutations using improved dynamic mutation detection (iDMD). Anal Chem 92(5): 3958-3965.
- Zhang Q, Yu H, Li Y, Yang X, Zhao X, et al. (2021) Applications of liquid biopsy in breast cancer: biomarkers, circulating tumor cells, and circulating tumor DNA. J Breast Cancer 24(1): 1-11.
- Sheng Y, Wang Y, Gu X, Bai T, Lu X, et al. (2020) Comparing circulating tumor cells and circulating tumor DNA in patients with colorectal cancer receiving chemotherapy: an exploratory study. Clin Colorectal Cancer 19(2): 106-113.
- Sanz Castleblanco Á, Burgos Panadero R, Carrión Álvarez L, Valdivia Bernal R, Salido Guadarrama I, et al. (2020) Circulating tumor DNA and liquid biopsy: opportunities, challenges and prospects. Clin Chim Acta 500: 80-92.
- Kato S, Uchida K, Kukita Y, Kumagai T, Nishino K, Daga H, et al. (2019) Prospective monitoring of tumor-derived DNA in cerebrospinal fluid and neuroimaging to detect occult leptomeningeal disease of lung cancer. Genome Med 11(1): 44. Wang Z, Luo X, Lu Y, Yang B, Zhang Y, Shi Y, et al. (2020) Liquid biopsy for gastric cancer: circulating tumor DNA and circulating tumor cells. Transl Oncol. 13(12): 100853.
- Lee M, Kim D, Han J, Kim H, Choi J, Kim Y, et al. (2021) Targeted sequencing and liquid biopsy approaches in lung cancer: a review Transl Lung Cancer Res10(3): 1407-1426.
- Alegre E, Fusco JP, Restrepo I, Mintz A, Torres-Roca JF (2020) Liquid biopsy in pancreatic cancer: current status and future directions. J Surg Oncol 121(6): 988-999.
- Bai H, Wu D, Wang Y, Zhao J, Zhang Z (2018) Liquid biopsy in tumors: opportunities and challenges. Ann Transl Med 6(17): 341.
- Wu C, Zhao J, Wang Y, Zhu X, Wang Z, Zhang Z, et al. (2015) Detection of circulating tumor DNA in peripheral blood of lung cancer patients using whole genome sequencing. Clin Cancer Res 21(9): 2096-2106.
- Li X, Liu L, Li L, Liang J, Sun M, Zhou J, et al (2021) The diagnostic value of liquid biopsy in oncogene-driven non-small cell lung cancer patients. Front Oncol 11: 642984.
- Kwapisz D. (2017) The first liquid biopsy test approved. Is it a new era of mutation testing for non-small cell lung cancer? Ann Transl Med 5(3): 46.
- Chaudhuri AA, Chabon JJ, Lovejoy AF, Newman AM, Stehr H, Azad TD, et al. (2017) Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov 7(12): 1394-1403.
- Schwaederle M, Husain H, Fanta PT, Piccioni DE, Kesari S, Schwab RB, et al. (2017) Detection rate of actionable mutations in diverse cancers using a biopsy-free (blood) circulating tumor cell DNA. Chae YK, Davis AA, Carneiro BA, et al. Concordance and discordance of somatic mutations and copy number changes between liquid and tissue biopsies in oncology. Mol Cancer Ther 16(5): 1073-1082.
- Pantel K, Alix Panabières C (2019) Liquid biopsy and minimal residual disease – latest advances and implications for cure. Nat Rev Clin Oncol 16(7): 409-424.
- O Leary B, Hrebien S, Beaney M, et al. (2019) Comparison of BEAMing and Droplet Digital PCR for Circulating Tumor DNA Analysis. Clin Chem 65(11): 1405-1413.
- Russo M, Siravegna G, Blaszkowsky LS, et al. (2016) Tumor Heterogeneity and Lesion-Specific Response to Targeted Therapy in Colorectal Cancer. Cancer Discov 6(2): 147-153.
- Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B (2011) Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci USA 108(23): 9530-9535.
- Wan JCM, Massie C, Garcia Corbacho J, et al. (2017) Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 17(4): 223-238.
- Heitzer E, Haque IS, Roberts CES, Speicher MR (2019) Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet 20(2): 71-88.
- Cohen JD, Li L, Wang Y, et al. (2018) Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359(6378): 926-930.
- Siravegna G, Marsoni S, Siena S, Bardelli A. (2017) Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 14(9): 531-548.
- Murtaza M, Dawson SJ, Tsui DWY, et al. (2013) Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 497(7447): 108-112.
- Bettegowda C, Sausen M, Leary RJ, et al. (2014) Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 6(224): 224ra24.
- Heitzer E, Ulz P, Belic J, et al. (2013) Tumor-associated copy number changes in the circulation of patients with prostate cancer identified through whole-genome sequencing. Genome Med 5(4): 30.
- Minciacchi VR, Freeman MR, Di Vizio D (2015) Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol 40: 41-51.
- Zocco D, Ferruzzi P, Cappello P, et al. (2019) Extracellular Vesicles as shuttles of tumor biomarkers and Anti-tumor drugs in Cancer Therapy. Int J Mol Sci 20(8): 1848.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.